溶酶体是细胞降解循环生物大分子物质的重要细胞器。它接收来自包括吞噬等多种囊泡运输途径转运的货物。降解后,氨基酸、糖、核苷酸等生物大分子经由溶酶体膜上转运蛋白运送至胞质中,供细胞再利用。而溶酶体需要经历再形成的过程维持其正常的稳态平衡。
近期,细胞生物学著名期刊The Journal of Cell Biology (JCB) 2019年第8期发表了威尼斯欢乐娱人城AⅤ大中国威尼斯欢乐娱人城AⅤ大中国杨崇林教授实验室的研究论文,题目是The amino acid transporter SLC-36.1 cooperates with PtdIns3P 5-kinase to control phagocytic lysosome reformation。该项研究结果以Article形式在线发表,并被选为封面文章。

JCB封面:吞噬溶酶体再形成的电镜图片

在微分干涉显微镜下,线虫胚胎细胞发生凋亡后呈现出特殊的纽扣状突起形态。杨崇林实验室通过正向遗传学筛选,获得了多个凋亡细胞呈凹坑状的突变体,经克隆发现是由于slc-36.1基因功能缺失突变导致。SLC-36.1蛋白在人类中的同源物是中性氨基酸转运蛋白SLC-36A1。SLC-36.1定位在细胞质膜和溶酶体膜上,而且它的氨基酸转运活性对于其功能是必须的。研究者用绿色荧光蛋白标记溶酶体膜蛋白LAAT-1,用红色荧光蛋白标记溶酶体基质蛋白NUC-1,在荧光显微镜追踪溶酶体在生理条件下的变化过程。在野生型线虫的胚胎中,随着吞噬溶酶体不断伸出丝状结构,并显示有新的溶酶体产生,而吞噬溶酶体的体积不断缩小并最终消失。但是,在slc-36.1突变体的胚胎中,吞噬溶酶体出丝的次数明显减少,并且吞噬溶酶体的体积在很长时间内都保持不变(下图)。因此,SLC-36.1对于溶酶体从吞噬溶酶体上再形成的过程是必需的.
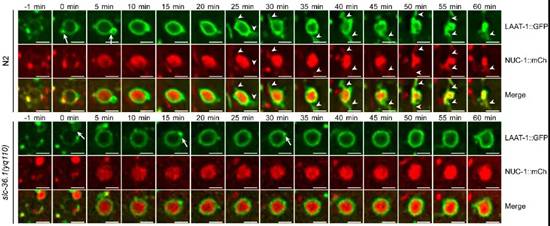
SLC-36.1为溶酶体从吞噬溶酶体上再形成所必需
该研究还发现PPK-3功能缺失同样会阻碍溶酶体从吞噬溶酶体上再形成。PPK-3是磷脂酰肌醇-3-磷酸激酶,能够催化磷脂酰肌醇-3-磷酸继续磷酸化,生成磷脂酰肌醇-3,5-二磷酸,已知磷脂酰肌醇-3,5-二磷酸对于膜分裂起着重要作用。进一步的研究表明,SLC-36.1直接与PPK-3相互作用,形成复合体,协同作用发挥调控作用。研究还发现,在溶酶体从自噬溶酶体上再形成的过程中,SLC-36.1-PPK-3复合物也发挥了调控作用。
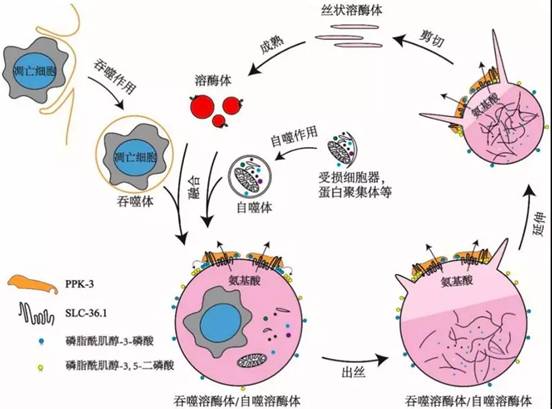
SLC-36.1-PPK-3复合物调控溶酶体再形成的模式图
综上所述,本项研究提供的实验证据指明了SLC-36.1-PPK-3复合物在吞噬溶酶体/自噬溶酶体的溶酶体再形成过程中起到了至关重要的调控作用。
据悉,云南生物资源保护与利用国家重点实验室、威尼斯欢乐娱人城AⅤ大中国及威尼斯欢乐娱人城AⅤ大中国为本研究的第一单位。实验室甘启文博士为本文第一作者,国家杰出青年科学基金获得者杨崇林教授为通讯作者。
原文链接:http://jcb.rupress.org/content/early/2019/06/21/jcb.201901074/tab-article-info?versioned=true
参考文献:
1. BISSIG, C., HURBAIN, I., RAPOSO, G. & VAN NIEL, G. 2017. PIKfyve activity regulates reformation of terminal storage lysosomes from endolysosomes. Traffic, 18, 747-757.
2. CHEN, C. C., SCHWEINSBERG, P. J., VASHIST, S., MAREINISS, D. P., LAMBIE, E. J. & GRANT, B. D. 2006. RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol Biol Cell, 17, 1286-97.
3. CHEN, D., XIAO, H., ZHANG, K., WANG, B., GAO, Z., JIAN, Y., QI, X., SUN, J., MIAO, L. & YANG, C. 2010. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science, 327, 1261-4.
4. CHEN, D., YANG, C., LIU, S., HANG, W., WANG, X., CHEN, J. & SHI, A. 2018. SAC-1 ensures epithelial endocytic recycling by restricting ARF-6 activity. J Cell Biol, 217, 2121-2139.
5. CHEN, Y. & YU, L. 2018. Development of Research into Autophagic Lysosome Reformation. Mol Cells, 41, 45-49.
6. LI, Y., XU, M., DING, X., YAN, C., SONG, Z., CHEN, L., HUANG, X., WANG, X., JIAN, Y., TANG, G., TANG, C., DI, Y., MU, S., LIU, X., LIU, K., LI, T., WANG, Y., MIAO, L., GUO, W., HAO, X. & YANG, C. 2016. Protein kinase C
controls lysosome biogenesis independently of mTORC1. Nat Cell Biol, 18, 1065-77.
7. LIU, B., DU, H., RUTKOWSKI, R., GARTNER, A. & WANG, X. 2012. LAAT-1 is the lysosomal lysine/arginine transporter that maintains amino acid homeostasis. Science, 337, 351-4.
8. LIU, K., XING, R., JIAN, Y., GAO, Z., MA, X., SUN, X., LI, Y., XU, M., WANG, X., JING, Y., GUO, W. & YANG, C. 2017a. WDR91 is a Rab7 effector required for neuronal development. J Cell Biol, 216, 3307-3321.
9. LIU, X., LI, Y., WANG, X., XING, R., LIU, K., GAN, Q., TANG, C., GAO, Z., JIAN, Y., LUO, S., GUO, W. & YANG, C. 2017b. The BEACH-containing protein WDR81 coordinates p62 and LC3C to promote aggrephagy. J Cell Biol, 216, 1301-1320.
10. MARTINA, J. A., DIAB, H. I., LISHU, L., JEONG, A. L., PATANGE, S., RABEN, N. & PUERTOLLANO, R. 2014. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal, 7, ra9.
11. MCCARTNEY, A. J., ZHANG, Y. & WEISMAN, L. S. 2014. Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. Bioessays, 36, 52-64.
12. MELENDEZ, A., TALLOCZY, Z., SEAMAN, M., ESKELINEN, E. L., HALL, D. H. & LEVINE, B. 2003. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science, 301, 1387-91.
13. NAGAI, T., IBATA, K., PARK, E. S., KUBOTA, M., MIKOSHIBA, K. & MIYAWAKI, A. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol, 20, 87-90.
14. SAMUELSON, A. V., CARR, C. E. & RUVKUN, G. 2007. Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes Dev, 21, 2976-94.
15. SATO, M., SAEGUSA, K., SATO, K., HARA, T., HARADA, A. & SATO, K. 2011. Caenorhabditis elegans SNAP-29 is required for organellar integrity of the endomembrane system and general exocytosis in intestinal
epithelial cells. Mol Biol Cell, 22, 2579-87.
16. SATO, T., MUSHIAKE, S., KATO, Y., SATO, K., SATO, M., TAKEDA, N., OZONO, K., MIKI, K., KUBO, Y., TSUJI, A., HARADA, R. & HARADA, A. 2007. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature, 448, 366-9.
17. VAN MEER, G. & SPRONG, H. 2004. Membrane lipids and vesicular traffic. Curr Opin Cell Biol, 16, 373-8.
18. WANG, M., TANG, C., XING, R., LIU, X., HAN, X., LIU, Y., WANG, L., YANG, C. & GUO, W. 2018. WDR81 regulates adult hippocampal neurogenesis through endosomal SARA-TGFbeta signaling. Mol Psychiatry.
19. YAMASHIRO, D. J. & MAXFIELD, F. R. 1984. Acidification of endocytic compartments and the intracellular pathways of ligands and receptors. J Cell Biochem, 26, 231-46.
20. ZHANG, D., ISACK, N. R., GLODOWSKI, D. R., LIU, J., CHEN, C. C., XU, X. Z., GRANT, B. D. & RONGO, C. 2012. RAB-6.2 and the retromer regulate glutamate receptor recycling through a retrograde pathway. J Cell Biol, 196, 85-101.


